Ozone Layer - Earth’s shield
-Navneet Kumar Gupta
The Earth's atmosphere is a thin layer of gases that surrounds it. It consists of oxygen, carbon di oxide, nitrogen, hydrogen, helium, ozone etc. These gases surround the Earth like a blanket. The atmosphere is one of the most important factors supporting the existence of life on Earth. But today, even the atmosphere is not untouched by pollution that is the result of human activities.
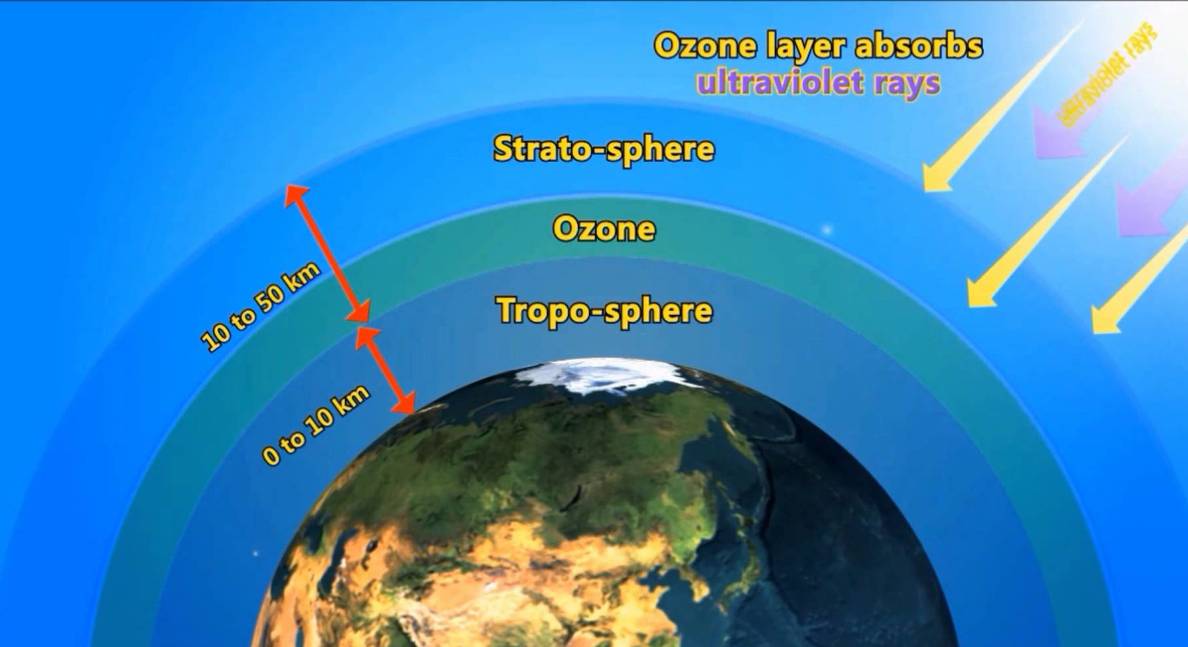
Ozone is a colourless, gaseous form of oxygen found in the Earth's atmosphere, primarily in the upper region known as the stratosphere, where it is naturally produced and destroyed. The chemical element oxygen normally forms a molecule containing two atoms (O2). But in the presence of ultra-violet light or an electrical spark in the air, oxygen can form a molecule containing three atoms (O3). The molecule of three oxygen atoms is called ozone. The oxygen molecule in the presence of the sun's rays breaks up into two independent oxygen atoms.
The free oxygen atoms combine with the other oxygen molecule to form the ozone molecule. This sequential gathering of ozone has been going on for the last couple of trillion years and has formed what is commonly known as the ozone layer. The unit of measure used to represent the amount of ozone above a particular position on the surface is the Dobson unit (DU), with one unit representing 0.01 mm of ozone compressed to one standard atmosphere. Therefore, there is typically 300 DU in a column of the normal atmosphere. G. M. B. Dobson was a British physicist who initiated the first regular monitoring of atmospheric ozone using spectrographic instruments in the 1920s.
Ozone-depleting substances:
Ozone gas is highly reactive in nature. Chlorofluorocarbon (CFC), Hydrochlorofluorocarbon (HCFC) and other chlorine compounds present in the atmosphere reacts with ozone to form chlorine molecule and release oxygen atom from ozone. The chlorine acts as a catalyst and converts ozone to an oxygen molecule. This depletion in the amount of ozone in the ozone layer is also termed as an ozone hole.
Ozone-depleting substances are often called halocarbons as they generally contain carbon, a halogen (e.g. chlorine, fluorine, bromine) and sometimes hydrogen. Specific groups of ozone-depleting substances include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), hydrobromofluorocarbons (HBFCs) or halons. These substances are mainly used in the refrigeration, air-conditioning and fire extinguishing sectors.
The various industrial activities releases different chemical like CFC, halons (A compound consisting of bromine, fluorine, and carbon), Carbon Tetrachloride etc. are responsible for increasing this ozone hole. A bigger hole is observed in the region of Antarctica in 2008. According to a report published in the World Meteorological Organization, the size of this ozone hole was 27 km till Sept 2008. It is an alarming trend that the size of the ozone hole is on the increase.
A proposal to ban the ozone-depleting substance (ODS) systematically was discussed in the Montreal Protocol in 1987. Some nations have prohibited the use of ozone-depleting substances like CFC, halons; Carbon Tetrachloride etc. and other nations are trying to do so. It’s our moral responsibility and duty to cut down our use of ODS. We must use ozone friendly substances (HFC-134a, HFC-152a, HC-290, HC-600a, etc.). Thus by our joint efforts, ozone will continue to protect us from the harmful UV rays and help to maintain the lively environment on Earth.
This year marks the 30th anniversary of the Vienna Convention for the Protection of the Ozone Layer, an important milestone in the protection of the ozone layer. The theme for the celebration of the anniversary and this year’s International Day for the Preservation of the Ozone Layer to be marked on 16 September is, “30 Years of Healing the Ozone Together.” The theme is supported by the slogan, “Ozone: All there is between you and UV.” The theme celebrates the collective efforts of the parties to the Vienna Convention and the Montreal Protocol in protecting the ozone layer over the past three decades, and the supporting slogan highlights the importance of the ozone layer in protecting life on Earth from the harmful effects of UV radiation.
As a result of concerted global efforts, the ozone layer is healing itself and is expected to recover by the middle of this century. In addition, up to 2 million cases of skin cancer may be prevented each year by 2030 and significant adverse effects on agriculture, wildlife, fisheries and materials have been avoided.
Ozone is even more precious than you might think. If our world’s entire atmosphere was at sea-level pressure and temperature, it would still be about 8,000 metres deep. Yet in all that, ozone would only occupy a layer just about 3mm deep. Ozone protects every living thing on Earth from harmful UV, so we are working together to maintain this remarkable natural resource.
The Montreal Protocol has been hailed as a prime example of successful international cooperation to protect the global commons. In addition to protecting the ozone layer, the Montreal Protocol is also contributing to protect the global climate. Political commitment by all Governments of the world and good governance have been fundamental to the milestones achieved by the Parties under the Protocol, which have in turn generated trust and confidence to meet further challenges in the coming years.
--------------------------------------
Ozone Layer Video
--------------------------------------
 Navneet Kumar Gupta is a science communicator working as a Project Officer (Edusat) in Vigyan Prasar-National institute of Science communication under the Department. of Science & Technology. Govt. Of India. He has deep interest in popular science writing for the general public through Print and electronic media. Besides his twelve books, he has written more than 200 popular science articles. He has edited/authored/co-authored more than 10 books. He have been awarded six National Awards including Rajbhasha Award, Ministry of Home Affairs, Government of India He has had a long stint as Associate Editor, VIPNET news - a popular science magazine. You may contact him at - ngupta@vigyanprasar.gov.in
Navneet Kumar Gupta is a science communicator working as a Project Officer (Edusat) in Vigyan Prasar-National institute of Science communication under the Department. of Science & Technology. Govt. Of India. He has deep interest in popular science writing for the general public through Print and electronic media. Besides his twelve books, he has written more than 200 popular science articles. He has edited/authored/co-authored more than 10 books. He have been awarded six National Awards including Rajbhasha Award, Ministry of Home Affairs, Government of India He has had a long stint as Associate Editor, VIPNET news - a popular science magazine. You may contact him at - ngupta@vigyanprasar.gov.in